Introduction: Why Hydrogen Production via Electrolysis Matters in 2025
As the world accelerates toward a clean energy future, hydrogen has emerged as a versatile, zero-emission fuel. Among various methods of hydrogen generation, electrolysis of water stands out as the most sustainable when powered by renewable sources. Electrolysis offers a pathway to produce green hydrogen, making it a cornerstone technology in the global effort to decarbonize industry, transportation, and power sectors.
What is Hydrogen Production via Electrolysis?
Understanding the Basics
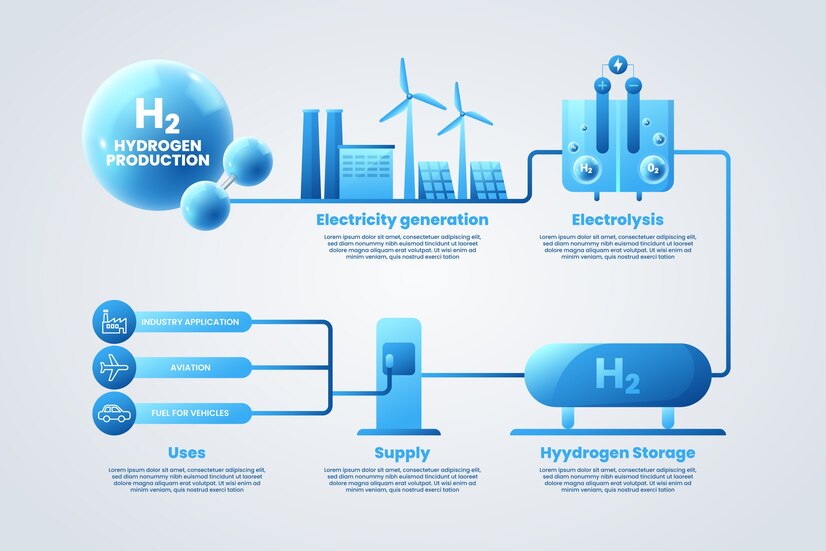
Electrolysis involves using electricity to split water (H₂O) into its components: hydrogen (H₂) and oxygen (O₂). The process takes place in a device called an electrolyzer, which typically consists of an anode, cathode, and electrolyte.
Chemical Reaction:
2H₂O → 2H₂ + O₂
When powered by renewable energy, this method results in zero carbon emissions—thus producing green hydrogen.
Key Benefits:
- Produces high-purity hydrogen
- Emits no greenhouse gases
- Easily integrated with solar and wind farms.
Types of Electrolyzers Used in 2025
1. Alkaline Electrolyzers
- Oldest and most mature technology
- Uses a liquid alkaline solution (e.g., KOH)
- Affordable but less efficient
2. Proton Exchange Membrane (PEM) Electrolyzers
- Uses a solid polymer membrane
- High efficiency and quick startup
- Preferred for mobility and modular applications
3. Solid Oxide Electrolyzers (SOEC)
- Operates at high temperatures
- Highest efficiency but still emerging
Comparison Table:
Type | Efficiency | Cost | Commercial Use |
Alkaline | Medium | Low | High |
PEM | High | Medium | Growing |
SOEC | Very High | High | Experimental |
How Electrolysis Supports Renewable Energy Integration
Hydrogen acts as an energy storage medium, making it ideal for balancing intermittent sources like solar and wind.
Use Cases:
- Solar Farms: Excess solar power during peak hours can drive electrolyzers.
- Wind Farms: Wind-generated electricity at night is ideal for continuous hydrogen production.
- Hybrid Systems: Electrolysis complements battery storage in microgrids.
Applications of Hydrogen from Electrolysis
1. Transportation
- Hydrogen Fuel Cell Vehicles (FCEVs)
- Hydrogen-powered buses, trains, and even aircraft
2. Industrial Use
- Steel manufacturing (hydrogen-based DRI)
- Fertilizer production (ammonia synthesis)
3. Power and Energy Storage
- Peak shaving in grid operations
- Seasonal energy storage
Challenges and Limitations
Technical:
- Electrolyzer durability
- Low efficiency at small scales
Economic:
- High upfront costs
- Need for supportive regulations
Infrastructure:
- Limited hydrogen pipelines
- Lack of refueling infrastructure
FAQ: Hydrogen Electrolysis
What is the most efficient method of hydrogen production?
Currently, PEM and solid oxide electrolysis are among the most efficient methods, especially when powered by renewables.
Is electrolysis cost-competitive in 2025?
With falling renewable electricity prices and government support, electrolysis is becoming increasingly competitive in many regions.
Can I produce hydrogen at home using electrolysis?
Yes, but only in small quantities. DIY electrolysis kits are available for educational purposes.
What industries are adopting hydrogen from electrolysis?
Steel, chemical production, transportation, and power storage are leading sectors.
Conclusion: The Future of Hydrogen Electrolysis Looks Bright
Hydrogen production via electrolysis is no longer a concept of the future—it’s a reality shaping today’s energy systems. As technology matures and costs fall, electrolysis stands to become the backbone of the hydrogen economy. From powering zero-emission vehicles to decarbonizing heavy industry, green hydrogen is a critical player in achieving net-zero emissions.
Want to explore electrolysis solutions for your organization? Contact our experts




